AIDS 2024 Conference Coverage
Preliminary findings from the phase III PURPOSE 1 trial revealed that lenacapavir (brand name Sunlenca), a long-acting injectable antiretroviral medication, demonstrated significant effectiveness in preventing HIV transmission among cisgender women in high-risk regions.
The groundbreaking research was presented by Linda-Gail Bekker, MBChB, PhD (Desmond Tutu HIV Centre at the University of Cape Town) at the International AIDS Conference, AIDS 2024, in Munich.
The data showed that in South Africa and Uganda, adolescent girls and young women saw a 100% reduction in HIV incidence with lenacapavir every 26 weeks compared to daily oral emtricitabine/tenofovir disoproxil fumarate (F/TDF – brand name Truvada).
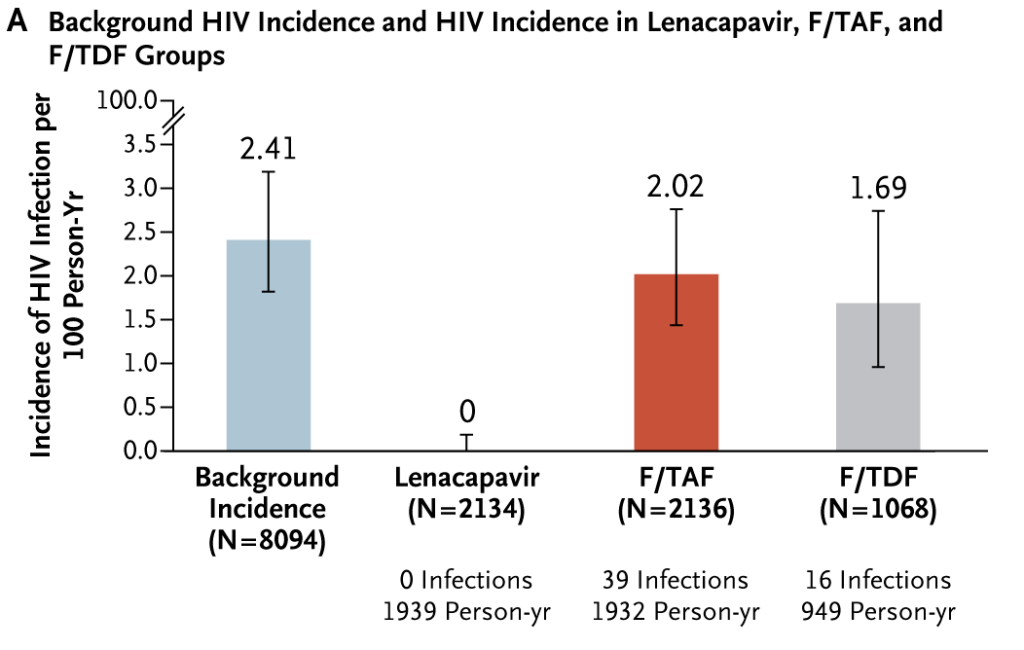
Because of these findings, the researchers opted to halt the randomised phase of the trial and gave all participants open-label lenacapavir.
Speaking on the results, Bekker said:
“Zero women receiving twice-yearly lenacapavir acquired HIV in PURPOSE 1”.
“The introduction of oral PrEP has changed the lives of more than 6 million people globally who have accessed it.”
“Despite PrEP’s promise, many young women have found uptake, daily adherence, and persistence to daily PrEP a social, emotional, and physical challenge. For them, we need new and diverse PrEP options.”
Whilst the results speak for themselves, implementing lenacapavir as PrEP may be stymied by the high price asked by Gilead Sciences.
Rochelle Walensky MD (Harvard Kennedy School of Government and Harvard Business School in Cambridge, Massachusetts) and Lindsey Baden MD (deputy editor of the New England Journal of Medicine) commented on the news: “That [it] is great news for science but not yet great for women”.
The pair also highlighted that whilst oral FTC/TDF costs around $50/year in the USA, lenacapavir sits at around $43,000 – making it a tough sell for patients, healthcare providers and governments alike.
Study methodology
A total of 5,338 teenage girls and young women who were HIV-negative at the start of the study were enrolled. The average age of the participants was 21 years old, with 2.3% being under 18. All participants were sexually active with male partners, not using PrEP, and had not been tested for HIV in the past 3 months. Most had completed secondary school. About 80% had been tested for HIV previously, with an average time since their last test being around 7 months, but only 6.3% had ever used any form of PrEP before.
Participants were randomised to receive either oral F/TAF, F/TDF or twice-yearly lenacapavir injections. Those who received oral PrEP received a placebo injection, and those on lenacapavir received placebo pills.
Participants were scheduled for check-ups at weeks 4, 8, and 13, followed by appointments every 13 weeks thereafter. They underwent laboratory tests, pregnancy screenings, and HIV testing. The rates of chlamydia, gonorrhoea, and other infections were notably high, averaging around 48 – 50 cases per 100 person-years, and were consistent among all three arms of the study. Those participants who tested positive for HIV during the study were referred and engaged in appropriate HIV care.
Reactions
Many attendees at the IAS AIDS 2024 conference commented on their excitement at the efficacy of lenacapavir as PrEP but also highlighted the challenges with access – especially in low-and-middle income countries.




Pingback: PrEP approved to prevent HIV in Japan - beyondpositive
Pingback: Twice-yearly lenacapavir 89% more effective than Truvada - beyondpositive
Pingback: Gilead to support generic lenacapavir in low-income nations - beyondpositive